科研进展
【ACS精准治疗】NEJM:高出血风险患者PCI术后的双抗策略
文章来源:IE-Learning急危重症学习平台发布时间:2021-12-30 12:27
【ACS精准治疗】NEJM:高出血风险患者PCI术后的双抗策略
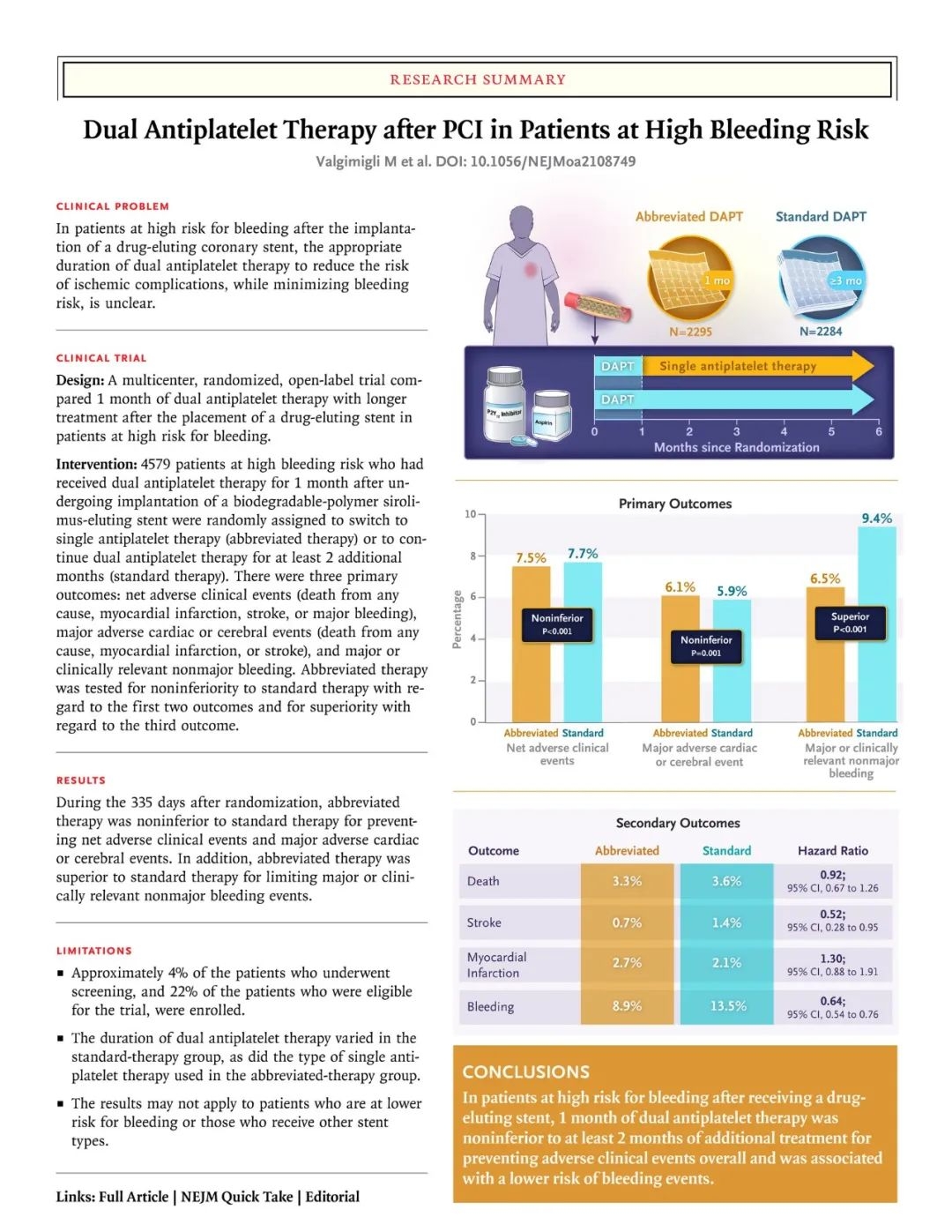 Background The appropriate duration of dual antiplatelet therapy in patients at high risk for bleeding after the implantation of a drug-eluting coronary stent remains unclear.
Background The appropriate duration of dual antiplatelet therapy in patients at high risk for bleeding after the implantation of a drug-eluting coronary stent remains unclear.
背景 对于置入药物洗脱冠状动脉支架术后出血风险高的患者,双重抗血小板治疗(DAPT)的适当持续时间尚不清楚。
Methods One month after they had undergone implantation of a biodegradable-polymer sirolimus-eluting coronary stent, we randomly assigned patients at high bleeding risk to discontinue dual antiplatelet therapy immediately (abbreviated therapy) or to continue it for at least 2 additional months (standard therapy). The three ranked primary outcomes were net adverse clinical events (a composite of death from any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (a composite of death from any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding; cumulative incidences were assessed at 335 days. The first two outcomes were assessed for noninferiority in the per-protocol population, and the third outcome for superiority in the intention-to-treat population.
方法 在置入生物可降解聚合物西罗莫司洗脱冠状动脉支架1个月后,Marco Valgimigli团队随机将高出血风险患者分配到立即停止双重抗血小板治疗组(简化治疗)或继续治疗至少2个月的治疗组(标准治疗)。在335天时评估累积发生率,排名前三的主要结果是净不良临床事件(包括全因死亡、心肌梗死、卒中或大出血)、重大不良心脑血管事件(包括全因死亡、心肌梗死或卒中)以及大出血或临床相关的非大出血事件。前两个结果在符合方案人群中评估非劣效性,第三个结果在意向治疗人群中评估优效性。
Results Among the 4434 patients in the per-protocol population, net adverse clinical events occurred in 165 patients (7.5%) in the abbreviated-therapy group and in 172 (7.7%) in the standard-therapy group (difference, −0.23 percentage points; 95% confidence interval [CI], −1.80 to 1.33; P<0.001 for noninferiority). A total of 133 patients (6.1%) in the abbreviated-therapy group and 132 patients (5.9%) in the standard-therapy group had a major adverse cardiac or cerebral event (difference, 0.11 percentage points; 95% CI, −1.29 to 1.51; P=0.001 for noninferiority). Among the 4579 patients in the intention-to-treat population, major or clinically relevant nonmajor bleeding occurred in 148 patients (6.5%) in the abbreviated-therapy group and in 211 (9.4%) in the standard-therapy group (difference, −2.82 percentage points; 95% CI, −4.40 to −1.24; P<0.001 for superiority).
结果 在符合方案的4434名患者中,简化DAPT治疗组有165名患者 (7.5%),标准DAPT治疗组有172名患者(7.7%)发生净不良临床事件(差异,-0.23 个百分点;95% 置信区间 [CI],-1.80 至 1.33;非劣效性 P<0.001)。简化治疗组中共有133名患者(6.1%),标准治疗组中有132名患者(5.9%)发生了严重的心脑血管不良事件(差异,0.11个百分点;95% CI,-1.29 至 1.51;非劣效性P=0.001)。在4579名意向治疗患者中,简化治疗组有148名患者(6.5%),标准治疗组有211名患者(9.4%)发生大出血或临床相关的非大出血事件(差异,-2.82 个百分点;95% CI,-4.40至-1.24;优势P <0.001)。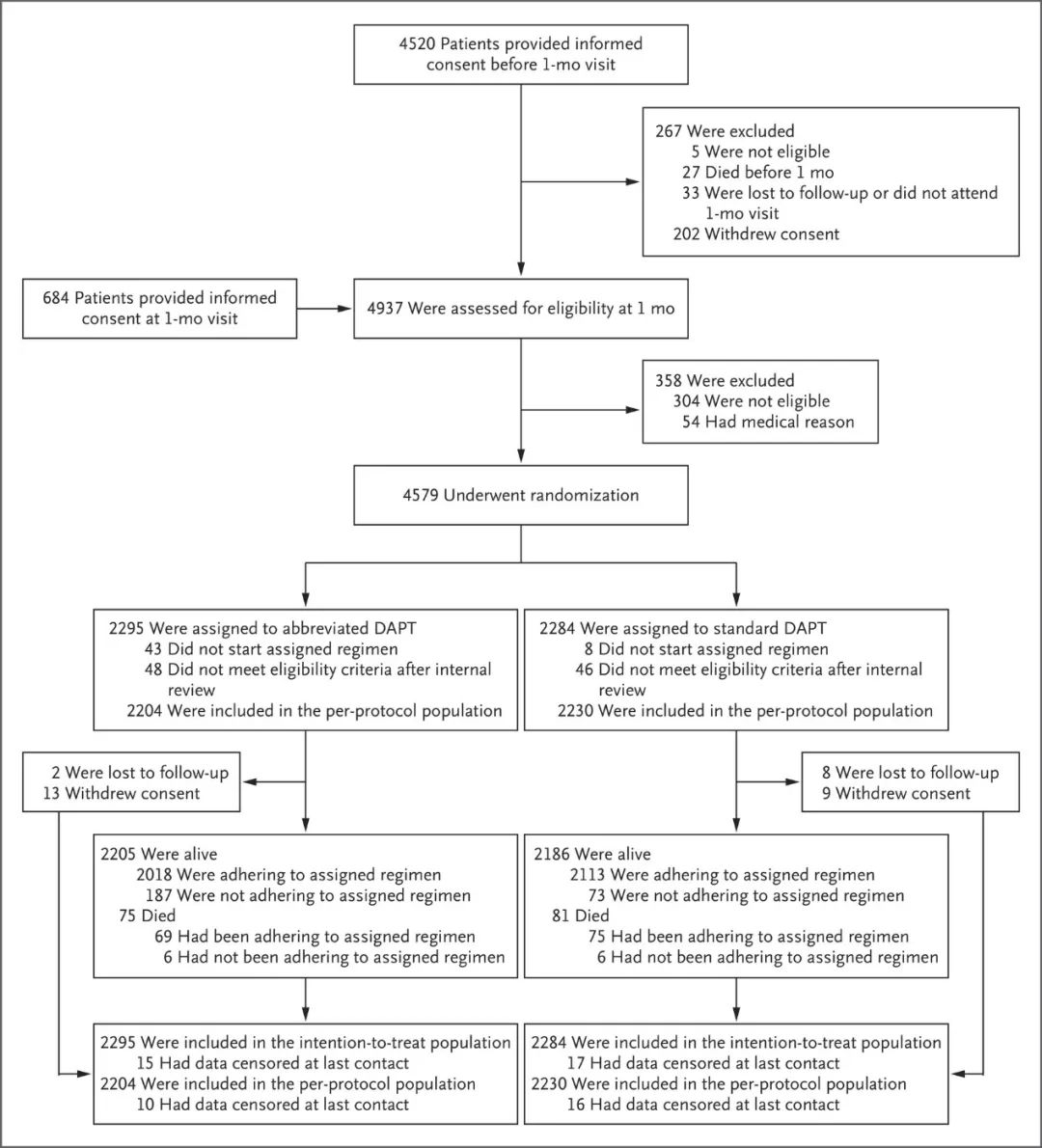 Figure 1. Randomization, Treatment, and Follow-up of the Patients.
Figure 1. Randomization, Treatment, and Follow-up of the Patients.
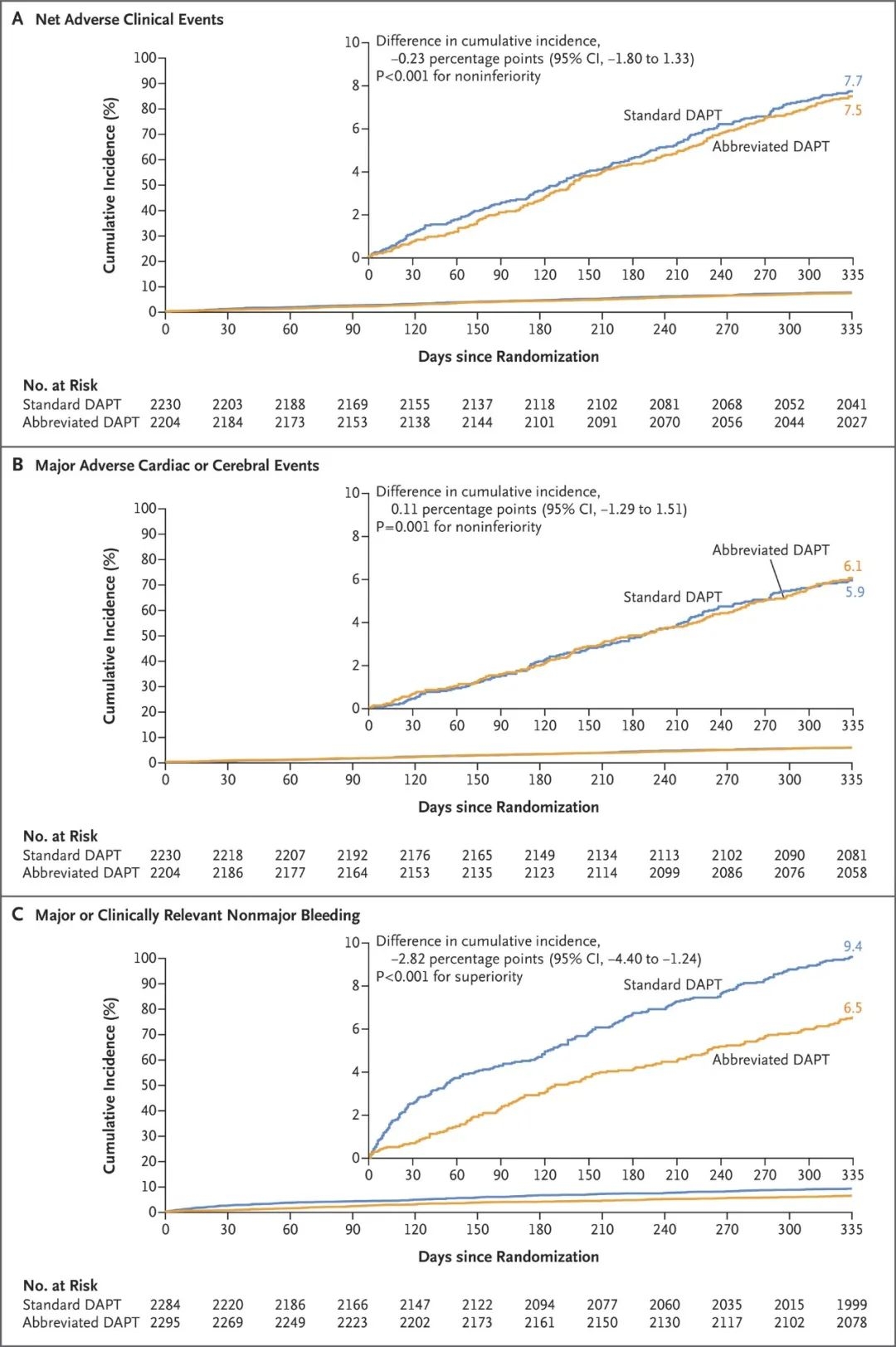
Figure 2 (facing page). Cumulative Incidence of Three Primary Composite Outcomes at 335 Days.
Conclusions One month of dual antiplatelet therapy was noninferior to the continuation of therapy for at least 2 additional months with regard to the occurrence of net adverse clinical events and major adverse cardiac or cerebral events; abbreviated therapy also resulted in a lower incidence of major or clinically relevant nonmajor bleeding.
结论 就净不良临床事件和主要不良心脑血管事件的发生率而言,1个月的双重抗血小板治疗与至少2个月的持续治疗无明显差异;缩短治疗周期使得大出血或临床相关的非大出血事件发生率降低。
全文链接:https://www.nejm.org/doi/10.1056/NEJMoa2108749
Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC; MASTER DAPT Investigators. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med. 2021 Oct 28;385(18):1643-1655.

背景 对于置入药物洗脱冠状动脉支架术后出血风险高的患者,双重抗血小板治疗(DAPT)的适当持续时间尚不清楚。
Methods One month after they had undergone implantation of a biodegradable-polymer sirolimus-eluting coronary stent, we randomly assigned patients at high bleeding risk to discontinue dual antiplatelet therapy immediately (abbreviated therapy) or to continue it for at least 2 additional months (standard therapy). The three ranked primary outcomes were net adverse clinical events (a composite of death from any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (a composite of death from any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding; cumulative incidences were assessed at 335 days. The first two outcomes were assessed for noninferiority in the per-protocol population, and the third outcome for superiority in the intention-to-treat population.
方法 在置入生物可降解聚合物西罗莫司洗脱冠状动脉支架1个月后,Marco Valgimigli团队随机将高出血风险患者分配到立即停止双重抗血小板治疗组(简化治疗)或继续治疗至少2个月的治疗组(标准治疗)。在335天时评估累积发生率,排名前三的主要结果是净不良临床事件(包括全因死亡、心肌梗死、卒中或大出血)、重大不良心脑血管事件(包括全因死亡、心肌梗死或卒中)以及大出血或临床相关的非大出血事件。前两个结果在符合方案人群中评估非劣效性,第三个结果在意向治疗人群中评估优效性。
Results Among the 4434 patients in the per-protocol population, net adverse clinical events occurred in 165 patients (7.5%) in the abbreviated-therapy group and in 172 (7.7%) in the standard-therapy group (difference, −0.23 percentage points; 95% confidence interval [CI], −1.80 to 1.33; P<0.001 for noninferiority). A total of 133 patients (6.1%) in the abbreviated-therapy group and 132 patients (5.9%) in the standard-therapy group had a major adverse cardiac or cerebral event (difference, 0.11 percentage points; 95% CI, −1.29 to 1.51; P=0.001 for noninferiority). Among the 4579 patients in the intention-to-treat population, major or clinically relevant nonmajor bleeding occurred in 148 patients (6.5%) in the abbreviated-therapy group and in 211 (9.4%) in the standard-therapy group (difference, −2.82 percentage points; 95% CI, −4.40 to −1.24; P<0.001 for superiority).
结果 在符合方案的4434名患者中,简化DAPT治疗组有165名患者 (7.5%),标准DAPT治疗组有172名患者(7.7%)发生净不良临床事件(差异,-0.23 个百分点;95% 置信区间 [CI],-1.80 至 1.33;非劣效性 P<0.001)。简化治疗组中共有133名患者(6.1%),标准治疗组中有132名患者(5.9%)发生了严重的心脑血管不良事件(差异,0.11个百分点;95% CI,-1.29 至 1.51;非劣效性P=0.001)。在4579名意向治疗患者中,简化治疗组有148名患者(6.5%),标准治疗组有211名患者(9.4%)发生大出血或临床相关的非大出血事件(差异,-2.82 个百分点;95% CI,-4.40至-1.24;优势P <0.001)。


结论 就净不良临床事件和主要不良心脑血管事件的发生率而言,1个月的双重抗血小板治疗与至少2个月的持续治疗无明显差异;缩短治疗周期使得大出血或临床相关的非大出血事件发生率降低。
全文链接:https://www.nejm.org/doi/10.1056/NEJMoa2108749
Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC; MASTER DAPT Investigators. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med. 2021 Oct 28;385(18):1643-1655.
